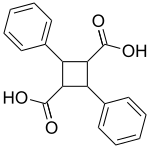
Truxillic acid
 | |
| Names | |
|---|---|
| IUPAC name 7,8′-Cyclo-8,7′-neolignane-9,9′-dioic acid | |
| Systematic IUPAC name 2,4-Diphenylcyclobutane-1,3-dicarboxylic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.022.478 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
Chemical formula | C18H16O4 |
| Molar mass | 296.322 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Truxillic acids are any of several crystalline stereoisomeric cyclic dicarboxylic acids with the formula (C6H5C2H2(CO2H)2. They are colorless solids. These compounds are obtained by the [2 + 2] photocycloadditions of cinnamic acid where the two trans alkenes react head-to-tail. The isolated stereoisomers are called truxillic acids.[1] The preparation of truxillic acids provided an early example of organic photochemistry.[2]

Cinnamic Acid CycloAddition
Occurrence and reactions
These compounds are found in a variety of plants, for example in coca.[3][4] Incarvillateine, an alkaloid from the plant Incarvillea sinensis, is a derivative of α-truxillic acid.
Upon heating, truxillic acids undergo cracking to give cinnamic acid.[5]
Isomers
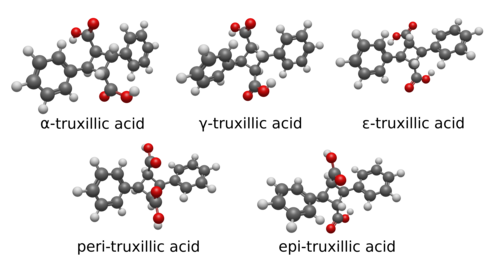
Truxillic acid can exist in five stereoisomers.[6][7]

| Isomer | a | b | c | d | e | f |
|---|---|---|---|---|---|---|
| α-truxillic acid (cocaic acid[8]) | COOH | H | H | C6H5 | H | COOH |
| γ-truxillic acid | COOH | H | H | C6H5 | COOH | H |
| ε-truxillic acid | H | COOH | C6H5 | H | H | COOH |
| peri-truxillic acid | COOH | H | C6H5 | H | COOH | H |
| epi-truxillic acid | COOH | H | C6H5 | H | H | COOH |
Below are the five stereoisomers of truxillic acid, called alpha, gamma, epsilon, peri, and epi. These are shown both in a 2D skeletal diagram with stereocenters indicated and a 3D rendering of the structural geometry of the isomers themselves.

See also
- Truxinic acids are isomers of the truxillic acids with phenyl groups on adjacent methyne centers.
References
- ^ Cohen, M. D.; Schmidt, G. M. J.; Sonntag, F. I. (1964). "Topochemistry. II. The photochemistry of trans-cinnamic acids". J. Chem. Soc.: 2000–2013. doi:10.1039/jr9640002000.
- ^ Roth, Heinz D. (1989). "The Beginnings of Organic Photochemistry". Angewandte Chemie International Edition in English. 28 (9): 1193–1207. doi:10.1002/anie.198911931.
- ^ Liebermann, C. (1888). "Ueber Cinnamylcocaïn". Berichte der Deutschen Chemischen Gesellschaft. 21 (2): 3372–3376. doi:10.1002/cber.188802102223.
- ^ Krauze-Baranowska, Miroslawa (2002). "Truxillic and truxinic acids-occurrence in plant kingdom". Acta Poliniae Pharmaceutica-Drug Research. 59 (5): 403–410. PMID 12602803.
- ^ Hein, Sara M. (2006). "An Exploration of a Photochemical Pericyclic Reaction Using NMR Data". Journal of Chemical Education. 83 (6): 940–942. Bibcode:2006JChEd..83..940H. doi:10.1021/ed083p940.
- ^ Stoermer, R.; Bachér, F. (1924). "Zur Stereoisomerie der Truxillsäuren und über die Auffindung der letzten Säure dieser Gruppe (VIII.)". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 57: 15–23. doi:10.1002/cber.19240570105.
- ^ Agarwai, O. P. (2011). Organic Chemistry Reactions and Reagents. Krishna Prakashan Media. ISBN 978-8187224655.
- ^ "ChemSpider ID 10218892". ChemSpider. Retrieved 15 October 2016.













