Neuropilin 1
| edit |
| Neuropilin 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB prikaz baziran na 1kex. | |||||||||||
| Dostupne strukture | |||||||||||
| 1KEX, 2QQI, 2QQM, 2QQN, 3I97, 4DEQ | |||||||||||
| Identifikatori | |||||||||||
| Simboli | NRP1; BDCA4; CD304; NP1; NRP; VEGF165R | ||||||||||
| Vanjski ID | OMIM: 602069 MGI: 106206 HomoloGene: 2876 GeneCards: NRP1 Gene | ||||||||||
| |||||||||||
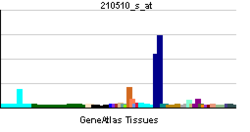
| Pregled RNK izražavanja | |||||||||||
 | |||||||||||
 | |||||||||||
 | |||||||||||
| podaci | |||||||||||
| Ortolozi | |||||||||||
| Vrsta | Čovek | Miš | |||||||||
| Entrez | 8829 | 18186 | |||||||||
| Ensembl | ENSG00000099250 | ENSMUSG00000025810 | |||||||||
| UniProt | O14786 | P97333 | |||||||||
| RefSeq (mRNA) | NM_001024628.2 | NM_008737.2 | |||||||||
| RefSeq (protein) | NP_001019799.1 | NP_032763.2 | |||||||||
| Lokacija (UCSC) | Chr 10: 33.47 - 33.63 Mb | Chr 8: 128.36 - 128.51 Mb | |||||||||
| PubMed pretraga | [1] | [2] | |||||||||
Neuropilin-1 je protein koji je kod ljudi kodiran NRP1 genom.[1][2][3] On je jedan od dva ljudska neuropilina.
NRP1 je za membranu vezan koreceptor receptora tirozinske kinaze za vaskularni endotelni faktor rasta (VEGF; MIM 192240) i članove familije semaforina (SEMA3A; MIM 603961). NRP1 ima više uloga u angiogenezi, navođenju aksona, ćelijskom opstanku, migraciji, i invaziji.[3]
Interakcije
Neuropilin 1 formira interakcije sa vaskularnim endotelnim faktorom rasta A.[1][4]
Reference
- ↑ 1,0 1,1 Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (April 1998). „Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor”. Cell 92 (6): 735–45. DOI:10.1016/S0092-8674(00)81402-6. PMID 9529250. Greška u referenci: Nevaljana oznaka
<ref>; naziv "pmid9529250" je zadan više puta s različitim sadržajem - ↑ Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M (November 1997). „Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III”. Neuron 19 (3): 547–59. DOI:10.1016/S0896-6273(00)80371-2. PMID 9331348.
- ↑ 3,0 3,1 „Entrez Gene: NRP1 neuropilin 1”.
- ↑ Mamluk, Roni; Gechtman Ze'ev, Kutcher Matthew E, Gasiunas Nijole, Gallagher John, Klagsbrun Michael (July 2002). „Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain”. J. Biol. Chem. (United States) 277 (27): 24818–25. DOI:10.1074/jbc.M200730200. ISSN 0021-9258. PMID 11986311.
Literatura
- Zachary I, Gliki G (2001). „Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family.”. Cardiovasc. Res. 49 (3): 568–81. DOI:10.1016/S0008-6363(00)00268-6. PMID 11166270.
- He Z, Tessier-Lavigne M (1997). „Neuropilin is a receptor for the axonal chemorepellent Semaphorin III.”. Cell 90 (4): 739–51. DOI:10.1016/S0092-8674(00)80534-6. PMID 9288753.
- Giger RJ, Urquhart ER, Gillespie SK, et al. (1999). „Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity.”. Neuron 21 (5): 1079–92. DOI:10.1016/S0896-6273(00)80625-X. PMID 9856463.
- Chen H, He Z, Bagri A, Tessier-Lavigne M (1999). „Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins.”. Neuron 21 (6): 1283–90. DOI:10.1016/S0896-6273(00)80648-0. PMID 9883722.
- Takahashi T, Nakamura F, Jin Z, et al. (1999). „Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors.”. Nat. Neurosci. 1 (6): 487–93. DOI:10.1038/2203. PMID 10196546.
- Rossignol M, Beggs AH, Pierce EA, Klagsbrun M (1999). „Human neuropilin-1 and neuropilin-2 map to 10p12 and 2q34, respectively.”. Genomics 57 (3): 459–60. DOI:10.1006/geno.1999.5790. PMID 10329017.
- Makinen T, Olofsson B, Karpanen T, et al. (1999). „Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1.”. J. Biol. Chem. 274 (30): 21217–22. DOI:10.1074/jbc.274.30.21217. PMID 10409677.
- Cai H, Reed RR (1999). „Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1.”. J. Neurosci. 19 (15): 6519–27. PMID 10414980.
- Takahashi T, Fournier A, Nakamura F, et al. (1999). „Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors.”. Cell 99 (1): 59–69. DOI:10.1016/S0092-8674(00)80062-8. PMID 10520994.
- Tamagnone L, Artigiani S, Chen H, et al. (1999). „Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates.”. Cell 99 (1): 71–80. DOI:10.1016/S0092-8674(00)80063-X. PMID 10520995.
- Gagnon ML, Bielenberg DR, Gechtman Z, et al. (2000). „Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity.”. Proc. Natl. Acad. Sci. U.S.A. 97 (6): 2573–8. DOI:10.1073/pnas.040337597. PMC 15970. PMID 10688880.
- Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G (2000). „Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected].”. J. Biol. Chem. 275 (24): 18040–5. DOI:10.1074/jbc.M909259199. PMID 10748121.
- Fuh G, Garcia KC, de Vos AM (2000). „The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1.”. J. Biol. Chem. 275 (35): 26690–5. DOI:10.1074/jbc.M003955200. PMID 10842181.
- Rossignol M, Gagnon ML, Klagsbrun M (2001). „Genomic organization of human neuropilin-1 and neuropilin-2 genes: identification and distribution of splice variants and soluble isoforms.”. Genomics 70 (2): 211–22. DOI:10.1006/geno.2000.6381. PMID 11112349.
- Simpson JC, Wellenreuther R, Poustka A, et al. (2001). „Systematic subcellular localization of novel proteins identified by large-scale cDNA sequencing.”. EMBO Rep. 1 (3): 287–92. DOI:10.1093/embo-reports/kvd058. PMC 1083732. PMID 11256614.
- Whitaker GB, Limberg BJ, Rosenbaum JS (2001). „Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121).”. J. Biol. Chem. 276 (27): 25520–31. DOI:10.1074/jbc.M102315200. PMID 11333271.
- Walter JW, North PE, Waner M, et al. (2002). „Somatic mutation of vascular endothelial growth factor receptors in juvenile hemangioma.”. Genes Chromosomes Cancer 33 (3): 295–303. DOI:10.1002/gcc.10028. PMID 11807987.
- p
- r
- u
PDB Galerija
|